
Building capacity to enhance ethics and human rights protection of participants in biomedical research
International Round Table
Introduction
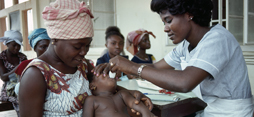
UNICRI, in collaboration with the Italian Medicines Agency, GCP Promotion Unit, GCP and Pharmacovigilance Inspectorate, has undertaken research on the procedures adopted in clinical trials involving the participation of human beings. In recent years, the practice of clinical trials involving human participants in developing countries has become widespread.
UNICRI and the Italian Medicines Agency, concerned by the proliferation of such activities, have undertaken extensive research into the ethical and legal implications of conducting biomedical research in countries with limited resources to monitor these practices, particularly in Africa. The results of this research were presented during an international Round Table event in Rome, on the 15-16 December 2008.
The overall objective of this research was to identify those countries most at need of education and training in ethical review and drug regulation. A survey was carried out on international training and education programmes and national legislation and guidelines regarding the protection of human participants in biomedical research.
Review processes and protocols used in clinical trials were scrutinised to examine the pressing ethical and legal issues affecting both investigators and participants.
The Round Table discussion provided a platform for internationally renowned experts in the field to present their own analyses of the various complex aspects of the phenomena, as well as to examine and debate the several issues raised by the research. International instruments developed by the United Nations and other relevant organisations regarding ethical and legal protections for human participants in clinical research trials were also discussed. It was agreed that increasing participants’ knowledge and understanding of the issues should be an objective.
Through this important opportunity to discuss issues which affect so many lives, UNICRI and the Italian Medicines Agency hoped to improve the current system of clinical trials and to protect vulnerable people from exploitation.
 unicri.it
unicri.it



