
Special Topics
Building capacity to enhance ethics and human rights protection of participants in biomedical research
 |
 |
|
UNICRI |
Italian Medicines Agency |
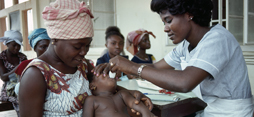
In the era of globalization, the spread of biomedical research with human subjects poses challenges at various various levels. The growing number of clinical trials conducted in developing settings,in Latin America, Africa and Asia requires total commitment from all stakeholders to ensure the correct application of internationally recognized Good Clinical Practices and adherence to the internationally recognized principles of ethics, human rights and justice.
In recent years, UNICRI, in collaboration with AIFA - the Italian Medicines Agency - has investigated the ethical and legal issues surrounding the conduct of clinical trials with human participants in developing countries, with particular regard to its criminal implications, such as the risks of fraudulent behavior, non compliance with the standards of ethical reviews and the lack of control on the quality of drugs and/or of the established protocols. In order to contribute to the creation of effective control mechanisms in this field, UNICRI and AIFA conducted a survey on the legislative framework related to the protection of participants in biomedical research. They also investigated the training, educational and capacity building activities carried out in the 53 African States which support the work of Ethical Review Boards and Inspectorates.
Deeply rooted in the Millennium Development Goals core objectives, UNICRI's policy of action-oriented research supports governments and the international community at large in strengthening human rights protection and the rule of law and tackling the threats that posed by crime to development and stability.
UNICRI believes that a proper legislative framework; its correct application; and adequate ethics training for professionals involved in research with human beings, are all key factors to ensuring the protection and promotion of human rights, safety and the well being of research participants all around the world.
Contacts
For further information please contact cte unicri.it
unicri.it




